Studying the thermal properties of liquid nitrogen by measuring its latent heat of vaporization.
Description
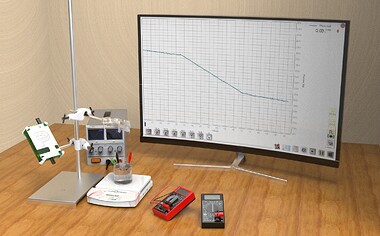
In this experiment, we present an intuitive setup to measure the latent heat of vaporization of liquid nitrogen and will learn about its thermal properties. The boiling point of liquid nitrogen is -196°C (77K) due to which it boils violently at room temperature. So, we will learn how to control this rate of boiling using electricity. We will also use a home-built load sensor to measure the mass loss of this boiling liquid nitrogen. Furthermore, we will be exposed to the safe handling of cryogens that are routinely used in physics.
Overview
- Introduction to calorimetry
- Measuring latent heat of vaporization
- Electrical to thermal energy conversion
- Cryogenic safety
- Error’s identification and propagation
- Graphical analysis of data
How Does it work?
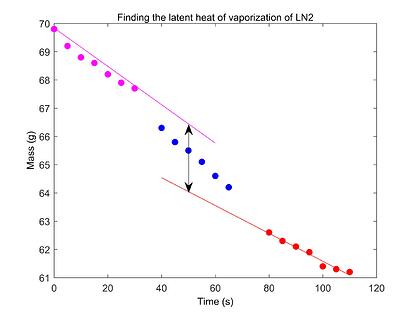
The latent heat of vaporization of liquid nitrogen is determined by measuring the rate of mass lost while it is being heated using a heater powered by a controlled DC power supply.
First, the background heat transfer of the liquid nitrogen with surroundings is quantifiably accounted for by recording the amount of mass lost while the heater is off. The heater is then turned on to increase the rate of evaporation. The power supplied to the heater is monitored using a multimeter and the rate of change of mass is recorded using a PhysLoad connected to PhysLogger, our data logging device. The collected data is used to determine the latent heat of the vaporization of nitrogen. A comparison of the experimental latent heat value with the theoretical figure is made to analyze possible error sources.
Parts Included
- PhysLoad
- PhysLogger
- Digital multimeter
- Cryogenic gloves
- Goggles
- PhysVolt
>You can get the PDF version of the brochure by clicking on this link.